How Verana Health Helps Elucidate Real-world Ophthalmic Treatment Patterns in nAMD and Glaucoma
Author:
Theodore Leng, MD, MS, Verana Health
Tracking real-world patient demographic profiles in ophthalmology presents a unique challenge to researchers seeking to better understand which types of patients receive care. The American Academy of Ophthalmology IRIS® Registry (Intelligent Research in Sight) allows large-scale collection of de-identified patient data by aggregating observational inputs from dozens of electronic health records systems.
Without data curation, the volume and complexity of the IRIS Registry would render this specialty-wide database cumbersome. By partnering with Verana Health—which uses its VeraQ® data engine to continually refine the real-world data found in the IRIS Registry into research-ready Qdata®—the Academy has facilitated analyses that will lend to a better understanding of real-world patient behavior.
The Value of Real-world Data in Ophthalmic Clinical Research: nAMD and Glaucoma
Two recent pieces in ophthalmic trade publications demonstrate how a curated IRIS Registry database leads to clarity in the field. The first, “Characteristics of Patients Started on Brolucizumab,” was written by Mathew W. MacCumber, MD, PhD, and appeared in Retinal Physician. The second was a blog post that I authored with Michael Mbagwu, MD, a Medical Director at Verana Health, titled “Stand-alone MIGS Procedures in the Real World.”
Anti–vascular endothelial growth factor (anti-VEGF) agents are the standard of care drug used to treat patients with neovascular age-related macular degeneration (nAMD); two on-label and one off-label anti-VEGF agent had commonly been used in clinical practice for approximately a decade. The FDA last approved an anti-VEGF agent for the treatment of nAMD in 2011—that is, until the fourth quarter of 2019, when the approval of brolucizumab (Beovu, Novartis) presented a new option for clinicians.
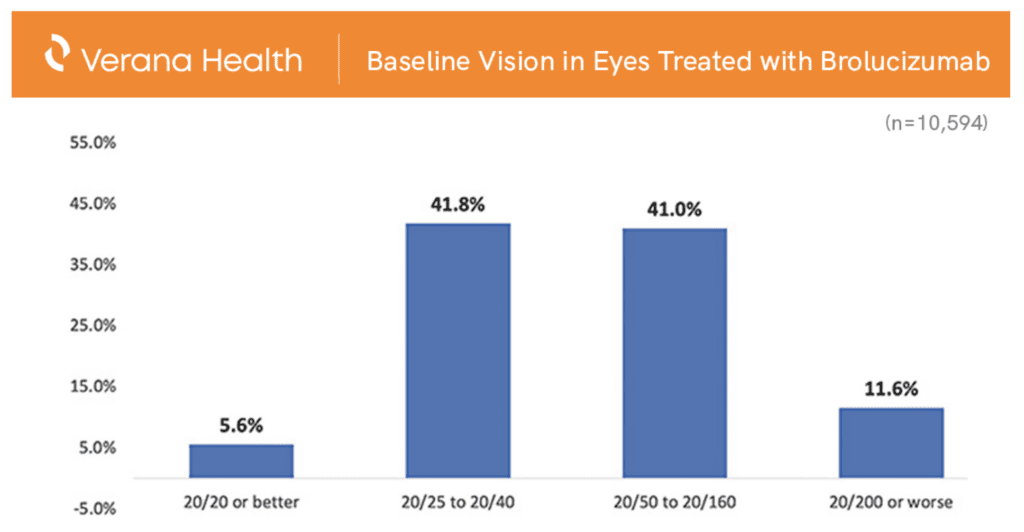
In an effort to understand which patient types received treatment with brolucizumab in the period immediately after its approval (and before COVID-related shutdowns induced aberrations in typical practice patterns), Dr. MacCumber and his research team turned to the IRIS Registry powered by Verana Health. In their analysis of real-world patients, they found that most eyes (83%) that received brolucizumab had visual acuity in the 20/25 to 20/160 range at baseline, with less than 12% seeing worse than 20/200 before switching to brolucizumab. Dr. MacCumber’s team also found that a majority (71%) of patients who were switched to brolucizumab had most recently undergone treatment with the anti-VEGF agent aflibercept (Eylea, Regeneron), with the remaining patients evenly split between the other two anti-VEGF agents commonly used in practice.
Learning about characteristics of real-world patients who were among the first to receive brolucizumab has implications beyond this particular drug. These data may inform future drug launches, elucidate treatment gaps, and guide marketing strategies for future technologies in this space. All of this would be impossible were it not for a curated specialty database.
The IRIS Registry also lent itself to research in patients with glaucoma. Minimally invasive glaucoma surgery (MIGS) is gaining popularity for its efficacy and safety. The iStent (Glaukos) and iStent Inject (Glaukos) are trabecular bypass MIGS devices approved for use by the FDA when combined with cataract surgery. However, some patients have received either of these trabecular bypass MIGS devices in standalone surgeries.
Dr. Mbagwu and I, along with a group of analysts, combed the IRIS Registry for information about how many patients underwent standalone vs combined trabecular bypass MIGS procedures. Because data in the IRIS Registry are organized coherently, we were able to quickly identify instances of each surgery type, and eliminated patient profiles that did not have enough data.
Among our findings were that nearly all (99%) of cases involving trabecular bypass MIGS devices between 2013 and 2020 occurred at the time of cataract surgery, leaving only 1% of cases occurring as an off-label standalone procedure. Patients who had standalone procedures had more severe glaucoma and were more likely to have a history of ocular surgery compared with those who underwent on-label combined procedures. These findings may help governing bodies, professional societies, and others make informed decisions regarding recommendations or regulations vis-à-vis trabecular bypass MIGS.
New analyses powered by the IRIS Registry and Verana Health are being conducted regularly. See a comprehensive list of research, here.

Let's Accelerate Research Together
To learn more about Verana Health, please fill out the information below and our team will follow up with you as soon as possible.
